One of the most peculiar things about Uranus and Neptune is their magnetic fields. Each of these planets has a hot mess of a magnetosphere, off-kilter and tilted wildly from the rotational axis in a way that's not seen in any other planet.
It's not entirely clear why, but thanks to a team of researchers from China and Russia we might have a new piece of the puzzle: a really weird, ionized form of water dubbed aquodiium that could exist deep in the extreme high-pressure interiors of these weird, icy worlds.
Aquodiium consists of a normal water molecule with two additional protons, giving it a net positive charge that – in sufficient enough quantities – could produce a planetary magnetic field like those of Uranus and Neptune.
Planetary magnetic fields extend far into the space around the planets produce them. They're generated, however, deep inside the planet by moving charges, although the precise mechanism can vary.
On Earth, it's the iron-nickel alloy sloshing around the core, rotating, convecting, and electrically conducting, converting all that kinetic energy into currents of electrons in what's called a dynamo. For Jupiter and Saturn, scientists think it's metallic hydrogen that provides a conduit for flowing electrons.
Earth, Jupiter, and Saturn have relatively tidy magnetic fields that resemble those of a huge bar magnet running down the planet's rotational axis, its field lines like a cage neatly connecting a north and south pole.
The magnetic poles of Uranus and Neptune, by contrast, are tilted 59 and 47 degrees, respectively, from their rotational axes, and the magnetic field lines are constantly morphing and shifting. And they're not actually centered on the planets' cores.
One possible explanation is the magnetic fields could be generated by an ionically conductive fluid, in which the ions are the charge carriers rather than the fluid acting as a conduit for electrons.
"The hydrogen surrounding Jupiter's rocky core at those [high-pressure] conditions is a liquid metal: It can flow, the way molten iron in the Earth's interior flows, and its electrical conductivity is due to the free electrons shared by all the hydrogen atoms pressed together," explains theoretical chemist, mineralogist, and physicist Artem Oganov of Skolkovo Institute of Science and Technology in Russia.
"In Uranus, we think that hydrogen ions themselves – i.e., protons – are the free charge carriers."
The question, then, is which ions? Some, like ammonium, are obvious possibilities. But could the planets' water molecules play a more significant role in the process as well?
Led by physicist Jingyu Hou of Nankai University in China, a team of researchers went back to first principles combined with models on the way molecules can evolve, delving into a concept called chemical hybridization.
This is when the orbital elements of an atom are mixed or combined to create an atom that can bond in new ways. There are different types of hybridization, but the one relevant here is sp3 hybridization, in which four orbitals form a tetrahedral arrangement around the central nucleus.
Each of the four points of the tetrahedron has either a lone electron capable of bonding with another atom, or an electron pair that can't form bonds with other atoms.
Oxygen has two single electrons and two electron pairs in its outer shell. If you attach a hydrogen atom to each of the available valence electrons, you get H2O – water.
Sometimes hydrogen without its electron – also known as a plain old proton – will attach to one of the electron pairs to form a molecule called a hydronium ion.
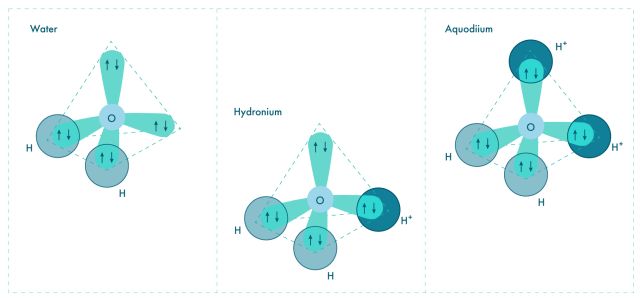
"The question was: Can you add yet another proton to the hydronium ion to fill in the missing piece? Such a configuration at normal conditions is energetically very unfavorable, but our calculations show there are two things that can make it happen," says physicist Xiao Dong of Nankai University.
"First, very high pressure compels matter to reduce its volume, and sharing a previously unused electron pair of oxygen with a hydrogen ion (proton) is a neat way of doing that: like a covalent bond with hydrogen, except both electrons in the pair come from oxygen. Second, you need lots of available protons, and that means an acidic environment, because that's what acids do – they donate protons."
The researchers conducted computational modeling, and, under conditions similar to those thought to exist inside Uranus and Neptune, this is what happened. At temperatures around 3,000 degrees Celsius (5,430 Fahrenheit), and pressures of 1.5 million atmospheres, protons attached to hydronium to form H4O2 – aquodiium.
It's still theoretical, of course. More detailed observations of the two outermost planets will be needed to investigate the possibility further; but the findings give us a new way to understand the blue oddballs that are Uranus and Neptune.
And they have implications for chemistry overall, too, representing, the researchers write, "an important addition to traditional physical and chemical theories such as the valence shell electron pair repulsion model, proton transfer, and acid-base theory."
The research has been published in Physical Review C.
