It's unclear how ALS gets started. There's no effective treatment, and the fast-progressing condition is terminal. So the discovery of a link to 'junk' (or non-functional) proteins in a new study is exciting for scientists.
ALS (amyotrophic lateral sclerosis), commonly called Lou Gehrig's disease after a famous baseballer with the condition, is a degenerative motor neuron disease involving the gradual death of nerves controlling muscles and movement.
One clue might be links between proteins rich in a specific amino acid and stress in an organelle called a nucleolus in the cells of patients with ALS. While previous research has found gene variants associated with ALS can produce these cell-stressing proteins, the exact cause of damage has yet to be identified.
A team led by researchers from the Spanish National Cancer Research Center (CNIO) used tests on cell cultures and mouse cells to further investigate the association between junk protein accumulation and cellular damage in ALS.
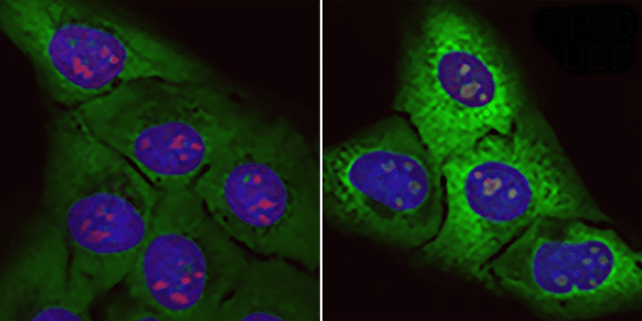
"In our work we report a new model that explains how nucleolar stress induces toxicity in animal cells, and we provide direct evidence that it accelerates aging in mammals," says Vanesa Lafarga, a molecular biologist at CNIO.
The researchers followed a complex biological path, starting with something we already know about hereditary ALS: it is characterized by an abundance of toxic peptides (chemical fragments) rich in the amino acid arginine.
The investigation linked that harmful abundance to a disruption in the cell's ability to manufacture key proteins the body needs to function. As the manufacturing breaks down, junk proteins – with no job to do – accumulate.
The biological manufacturing machines involved are called ribosomes, and there's an important connection to another group of rare diseases called ribosomopathies, where something similar happens. Knowing that these diseases share some characteristics with ALS should help us understand it more.
This research showed that the damaging effects of these junk proteins – in terms of cell messaging and repair, for example – match up well with what's previously been seen with ALS.
"These proteins end up collapsing the cellular clearance systems, which finally leads to the death of the motor neurons," says Óscar Fernández-Capetillo, a molecular biologist and vice director at CNIO.
We're still in the very early stages here, as these connections have only just been found. However, this knowledge could be used further down the line to tackle the root causes of conditions such as ALS.
Since nucleolar stress also triggers certain changes that indicate accelerated biological aging, or the speed at which cells show signs of wear and tear and eventually die, the discovery could have applications beyond treatment of ALS.
"We are in the first steps to see if we can give a therapeutic angle to these findings," says Fernández-Capetillo.
"We must find ways to reduce the production of ribosomes so that waste decreases, while still keeping a sufficient number to guarantee the correct functioning of the cells."
The research has been published in Molecular Cell.