Your intestines are likely littered with tiny plastic specks, a now-common condition for humans and many other animals around the planet. The pieces are minuscule, but as researchers report in a new study, they may pose an outsized danger to human health.
By testing how microplastics affect human intestinal organoids (small, self-organized tissue cultures that mimic real intestines), researchers found signs of potential inflammatory effects, including the release of cytokines linked to human inflammatory bowel disease (IBD).
This is significant, researchers explain, because while we know microplastics (along with even smaller nanoplastics) can accumulate in our intestines and other body tissues, we still know very little about how they affect our health.
That's partly because plastic particles have spread so thoroughly so quickly, swirling from obscurity to ubiquity within just a few human generations.
Formed as plastic products break down into smaller pieces over time, the particles can be incredibly durable and mobile, allowing them to end up seemingly anywhere on Earth.
Microplastics have pervaded environments and food webs worldwide, from cities and farms to oceans and ice sheets. We unwittingly eat, drink, and inhale them on a regular basis, with the average person now ingesting an estimated 74,000 tiny plastic particles every year.
"We know that particulate plastic is everywhere in the environment, and it has been found in human intestines and other tissues, like blood, and even in the brain and placenta," says study co-author Ying Chen, a Tufts University biomedical engineer.
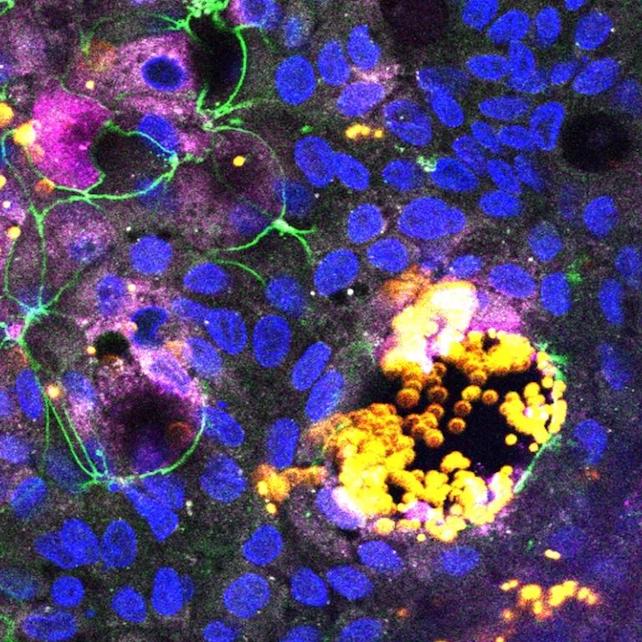
Microplastics have become so widespread that scientists hoping to study their health effects in humans now struggle to find unaffected populations who can serve as control groups, the researchers note.
And while previous research has found a correlation between microplastics and IBD, evidence of causation remains elusive. Animal studies offer mixed results, showing a buildup of plastic particles in the intestines and other tissues, but no clear answer on toxicity or inflammation.
So Chen and her colleagues tried a different approach for the new study, using human intestinal organoids to help them learn what microplastics might do in a real human gut.
"The use of organoids allows us to study in detail the mechanisms of absorption and potential pathways to disease in a way that could help us make sense of the variable results in the literature up until now," Chen says, "and have a more direct tissue model for potential effects of plastic particles on humans."
The researchers created their model of a human gut by prompting stem cells (which were derived from other organoids) to differentiate into the various cell types that naturally occur in the walls of actual human intestines.
They wanted a ratio of cells similar to real intestinal walls, hoping to simulate the complex environment in which cells perform critical jobs like absorption, hormone production, mucus secretion, and inflammatory responses, among others.
"It's a significant step up from simpler cell models that often included only one or a few cell types, some of which were derived from cancer cells that might not demonstrate natural responses," Chen says.
The experiments showed different cell types absorbing different sizes of particles. The smallest nanoplastics were taken in by epithelial cells, which line the interior walls of the intestines, while microfold cells – which play a role in the gut's immune response – absorbed larger particles and sent them into the intestinal tissue.
The intestinal model only sustained damage when microfold cells were present and when there were higher concentrations of small plastic particles. According to the authors, the damage suggests microplastics could contribute to the formation of intestinal lesions.
The study found that higher levels of nanoplastics also triggered the organoid to release inflammatory cytokines. These proteins are part of a normal immune response but may also play a role in diseases like IBD when something disrupts their natural balance.
This, too, happened only in the presence of microfold cells, suggesting they may help mediate any potential harm from microplastics in the intestines.
More research is needed to flesh out these findings and to clarify how different types, sizes, and amounts of microplastics can affect our guts. Still, this study offers a potentially valuable model for demystifying the danger.
"The results in this study suggest that using human cell organoids could be an effective means to better understand the potential toxicity of microplastics and nanoplastics and environmental particles in general," says co-author David Kaplan, professor of engineering at Tufts University.
The study was published in Nanomedicine.
