Semaglutide, the active ingredient in brand medicines like Ozempic and Wegovy, could raise the risk of a relatively rare eye disease that is a leading cause of vision loss, according to a retrospective study.
The research was led by scientists at Harvard University, and it was driven by anecdotal reports from doctors in Boston, who had three patients in their practice taking semaglutide suffer vision loss in just one week.
The follow-up analysis is based on the health data of 16,827 patients, 710 of whom were diagnosed with type 2 diabetes and 979 of whom were overweight or had obesity.
The authors of the study argue their findings should be viewed as "significant but tentative".
When participants were matched based on their age and sex, researchers found those treated with semaglutide in the past six years had a "substantially increased risk" of developing a disorder called non-arteritic anterior ischemic optic neuropathy (NAION).
NAION describes a condition that impacts roughly 10 in 10,000 people, and in simple terms, it is marked by decreased blood flow to the front of the optic nerve, which transmits visual information from the retina to the brain. Over time, this can lead to swelling and sometimes cause damage such as sudden and permanent vision loss.
In the current analysis, 11 percent of patients treated with semaglutide for diabetes in the past six years went on to develop NAION, compared to just 3 percent of their counterparts treated with other diabetic medications.
What's more, 7 percent of patients using semaglutide for weight loss went on to develop NAION, compared to 1 percent of matched peers using other weight loss medications.
"The use of these drugs has exploded throughout industrialized countries and they have provided very significant benefits in many ways, but future discussions between a patient and their physician should include NAION as a potential risk," says neuro-ophthalmologist Joseph Rizzo from Massachusetts Eye and Ear Hospital at Harvard University.
"It is important to appreciate, however, that the increased risk relates to a disorder that is relatively uncommon."
According to the North American Neuro-Ophthalmology Society, the loss of vision from NAION is typically painless and incomplete. It tends to start with a motionless gray or dark spot in the top or bottom half of one eye's vision.
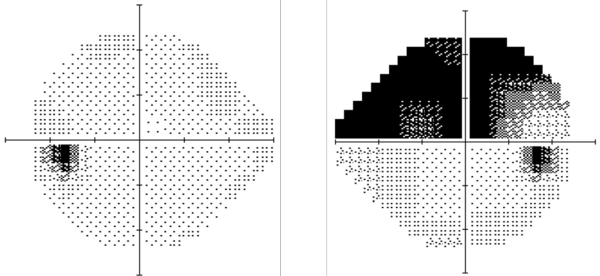
In extreme situations, losses to vision can occur within hours of onset, and while this effect can worsen in the following weeks, once the optic nerve's swelling goes down after a month or two, there can be some visual improvement. Repeat attacks are rare.
Those who are overweight or have type 2 diabetes are known to be at a greater risk of NAION in general, but the current analysis found a key difference in this risk factor when patients were treated with semaglutide.
Like any medicine, semaglutide comes with risks and benefits, but this novel drug, which was first approved by the US Food and Drug Administration (FDA) to treat diabetes in 2017, is still very new to science.
Now that semaglutide is also approved for weight loss at a higher dose, prescriptions have exploded into the millions, and researchers are learning about possible side effects in real time. Patient anecdotes online or in the clinic are often the driving force for further research.
Researchers at Harvard T.H. Chan School of Public Health, Brigham and Women's Hospital, and Massachusetts Eye and Ear Hospital, agree that "even more expansive use of these drugs seems likely given their medical benefits and widespread popularity."
"If true," they add, "our data anticipate increasing numbers of NAION cases related to this class of drugs."
Further research is needed to confirm why semaglutide might increase the risk of developing NAION. The optic nerve is known to host receptors that semaglutide can attach to, but the current research did not adequately address all the confounding factors that could be playing a role in the association.
"Future studies are needed to examine these questions in a much larger and more diverse population," says Rizzo.
"This is information we did not have before and it should be included in discussions between patients and their doctors, especially if patients have other known optic nerve problems like glaucoma or if there is pre-existing significant visual loss from other causes."
The study was published in JAMA.