We've just identified a brand new isotope of the rarest element in our planet's crust.
With 85 protons and 105 neutrons, 190astatine is the lightest isotope of astatine discovered yet, and could help physicists better understand the process of alpha decay, and the structure and limitations of atomic nuclei.
"The studies of new nuclei are important for understanding the structure of atomic nuclei and the limits of known matter," says physicist Henna Kokkonen of the University of Jyväskylä in Finland.
Astatine is highly radioactive, and extremely unstable. It only occurs in nature as a sort of stepping stone, a product of the decay of heavier elements that in turn rapidly decays into lighter elements. The most stable isotope of astatine is 210astatine, which has a half-life of just over 8 hours; most of its isotopes have half-lives less than a few seconds.
This is why it's so rare in nature, with barely a single gram present on our planet at ony one moment. It forms, it poofs away again, ejecting protons and neutrons until it lands in a stable form, an element such as bismuth or radon. Being so short-lived, its properties are largely derived by inference and are not known with any certainty.
We don't even know for sure if it's a halogen or a metalloid. It's a pretty weird element, no bones about it. But studying it can help us not only understand the element itself, but the deformation of nuclei of different isotopes, and radioactive decay.
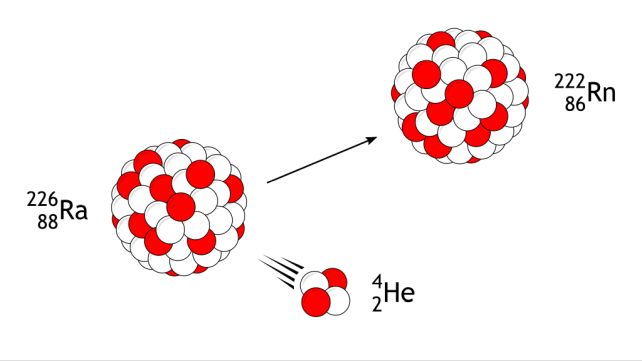
The research was undertaken using an apparatus called a gas-filled recoil separator, used to run fusion-evaporation experiments. This is when heavy ions are accelerated into target nuclei to fuse into heavier elements, which then decay via the alpha process, ejecting alpha particles consisting of two protons and two neutrons (helium, basically) until they stabilize.
The researchers fired 84strontium into target silver atoms, and studied the resulting decay products. They weren't looking for 190astatine, but 190astatine is what they found.
"In my thesis, I analyzed experimental data among which the new isotope was found," Kokkonen explains.
Previously, the most neutron-deficient isotope of the element we knew of was 192astatine. The researchers analyzed the new discovery, and compared it to predictions of atomic mass models to see if it can tell us anything new about astatine.
It seems pretty consistent with what we know about the element, and alpha decay. The isotope has a half-life of just 1 millisecond, and an energy level of 7,750 kiloelectronvolts, which is fairly normal. The decay was also unhindered, energetically; this just means it was immediate, rather than delayed, which can happen in some radioactive isotopes of heavy elements.
Not bad at all for a Master's thesis.
"During my thesis process and summer internships I got to know the Nuclear Spectroscopy group's work," Kokkonen says. "Now I am very happy to work in the group towards my Ph.D. degree."
The findings have been published in Physical Review C.