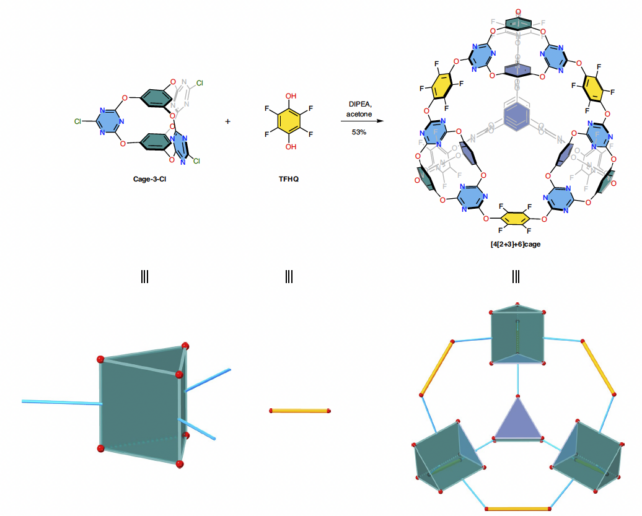
A 'cage of cages' is how scientists have described a new type of porous material, unique in its molecular structure, that could be used to trap carbon dioxide and another, more potent greenhouse gas.
Synthesized in the lab by researchers in the UK and China, the material is made in two steps, with reactions assembling triangular prism building blocks into larger, more symmetrical tetrahedral cages – producing the first molecular structure of its kind, the team claims.
The resulting material, with its abundance of polar molecules, attracts and holds greenhouse gasses such as carbon dioxide (CO2) with strong affinity. It also showed excellent stability in water, which would be critical for its use in capturing carbon in industrial settings, from wet or humid gas streams.
"This is an exciting discovery," says Marc Little, a materials scientist at Heriot-Watt University in Edinburgh and senior author of the study, "because we need new porous materials to help solve society's biggest challenges, such as capturing and storing greenhouse gasses."
Although not tested at scale, lab experiments showed the new cage-like material also had a high uptake of sulfur hexafluoride (SF6), which according to the Intergovernmental Panel on Climate Change, is the most potent greenhouse gas.
Where CO2 lingers in the atmosphere for 5–200 years, SF6 can hang about for anywhere between 800 to 3,200 years. So although SF6 levels in the atmosphere are much lower, its extremely long lifetime gives SF6 a global warming potential of around 23,500 times that of CO2 when compared over 100 years.
Removing large amounts of SF6 and CO2 from the atmosphere, or stopping them from entering it in the first place, is what we urgently need to do to reign in climate change.
Researchers estimate that we need to extract around 20 billion tons of CO2 each year to cancel out our carbon emissions that are only trending upwards.
So far, carbon removal strategies are removing about 2 billion tons per year, but that's mostly trees and soils doing their thing. Only about 0.1 percent of carbon removal, around 2.3 million tons per year, is thanks to new technologies such as direct air capture, which uses porous materials to soak up CO2 from the air.
Researchers are busy devising new materials to improve direct air capture to make it more efficient and less energy-intensive, and this new material could be another option. But to avert the worst impacts of climate change, we need to reduce greenhouse gas emissions faster than these nascent technologies currently can.
Nevertheless, we need to throw everything we can at this global problem. Creating a material of such high structural complexity wasn't easy though, even if the precursor molecules technically assemble themselves.
This strategy is called supramolecular self-assembly. It can produce chemically interlocked structures from simpler building blocks, but it takes some fine-tuning because "the best reaction conditions are often not intuitively obvious," Little and colleagues explain in their published paper.
The more complex the final molecule, the harder it becomes to synthesize and more molecular 'scrambling' could occur in those reactions.
To get a handle on those otherwise invisible molecular interactions, the researchers used simulations to predict how their starter molecules would assemble into this new type of porous material. They considered the geometry of potential precursor molecules, and the chemical stability and rigidity of the final product.
Aside from its potential to absorb greenhouse gasses, the researchers suggest their new material could also be used to remove other toxic fumes from the air, such as volatile organic compounds, which easily become vapors or gasses from surfaces including the inside of new cars.
"We see this study as an important step towards unlocking such applications in the future," Little says.
The study has been published in Nature Synthesis.