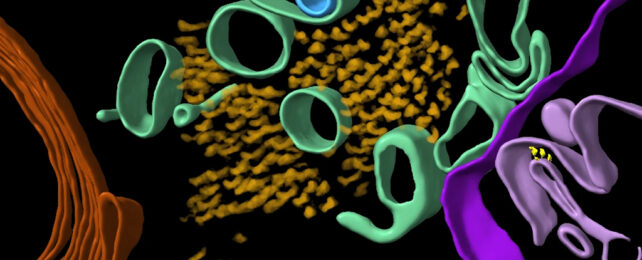
A new study has revealed for the first time the molecular structure of brains affected by Alzheimer's disease.
Researchers have produced 3D models of proteins in the brain, including two proteins in particular that are associated with Alzheimer's: beta-amyloid and tau.
As scientists continue to work towards treatments for the neurodegenerative disease, it's important to understand as much as we possibly can about it.
Clumps of these proteins in the brain are either a cause of Alzheimer's or a consequence of it – we're not quite sure which yet – and thanks to a team from the University of Leeds in the UK, we now have a very detailed look at how they are arranged, right down to the tiniest, microscopic details.
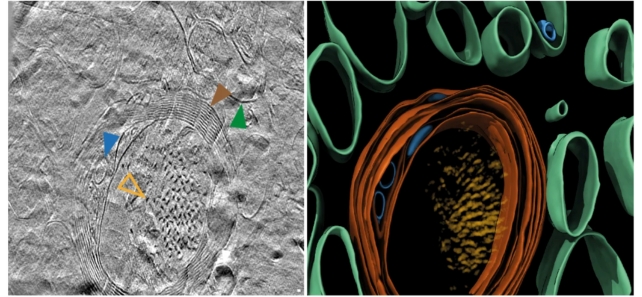
"This first glimpse of the structure of molecules inside the human brain offers further clues to what happens to proteins in Alzheimer's disease," says neuroscientist René Frank from the University of Leeds.
"But [it] also sets out an experimental approach that can be applied to better understand a broad range of other devastating neurological diseases."
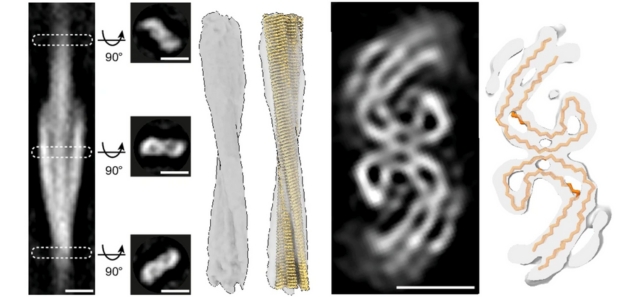
The researchers used a number of advanced imaging techniques to scan postmortem brain tissue from Alzheimer's patients, including cryo-electron tomography (cryoET) – which uses readings from beams of electrons to map out 3D structures of tissue immobilized at very low temperatures.
CryoET allows imaging without chemical fixation or dehydration disrupting the structure of biological tissue, meaning scientists can now reconstruct 3D volumes of tissue at resolutions a million times smaller than a grain of rice.
"Light microscopic characterization of amyloid in Alzheimer's disease brain has formed the basis of diagnostic and disease classification," write the researchers in their published paper.
"The in situ structure of amyloid in the human brain is unknown."
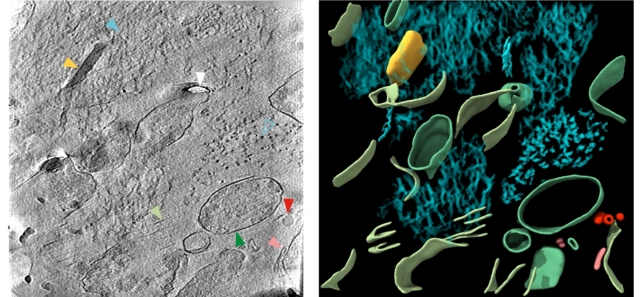
By getting such a close look at these proteins, the hope is that we can better understand how the clumps are formed and how they're impacting the brain.
In beta-amyloid proteins, a mixture of microscopic thread-like structures called fibrils and other structures were found. In tau proteins, there were clusters of filaments in straight lines, although the arrangement seems to vary depending on where the proteins are in the brain.
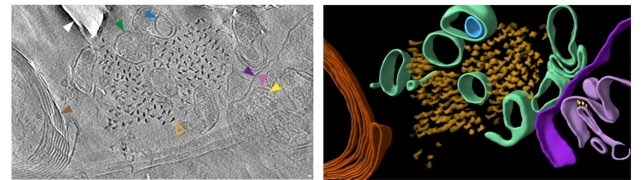
While clusters were similar to each other, there were differences across spatial organization, in terms of the way the tau filaments were oriented and twisted, as well as in the size of the beta-amyloid fibrils.
This is the first look we've had at these proteins at this level of detail, and it's too early to say anything about the significance of what's been revealed. Now that the technique has been shown to work, it can be tried on tissue from a wider range of brain donors.
That will reveal more about how these different proteins look at the different points of Alzheimer's progression – and by comparing the structures across time, we should be able to see how the disease develops.
In fact, the team behind the new study thinks that this approach could be useful in analyzing the root causes of all kinds of neurodegenerative diseases, so we can expect to hear more about it in the future.
"Larger cohorts of diverse Alzheimer's disease donors, across different brain regions and at earlier stages of Alzheimer's disease, may reveal how the spatial organization of amyloid of different structures relates to individual neuropathological profiles," write the researchers.
"It will also be important to apply these approaches to other neurodegenerative diseases, many of which share related, or overlapping, types of amyloid neuropathology."
The research has been published in Nature.