A 58-year-old man in the United States is the first person in the world to have had his failing heart replaced with a temporary, titanium blood-pumper.
The metal organ looks like it was made for the Tin Man, but it was actually designed by the medical device company BiVACOR to replace the full function of a flesh-and-blood human heart for as long as possible.
BiVACOR's Total Artificial Heart (TAH) is not designed to beat like a real one. But even without flexible chambers or pumping diaphragms, BiVACOR claims it is powerful enough to sustain a man during exercise, and small enough to suit most men and women.
The double-chambered device is about the size of a fist and is virtually unbreakable, resisting corrosion and mechanical wear.
The only moving part is hidden within; a single, magnetically levitating rotor which pumps blood to the lungs and on to the rest of the body. Because the spinning rotor does not make contact with any other surface, there is no chance that friction will cause damage over time.
The whole device is powered by a small, external, portable controller that exits through the stomach.
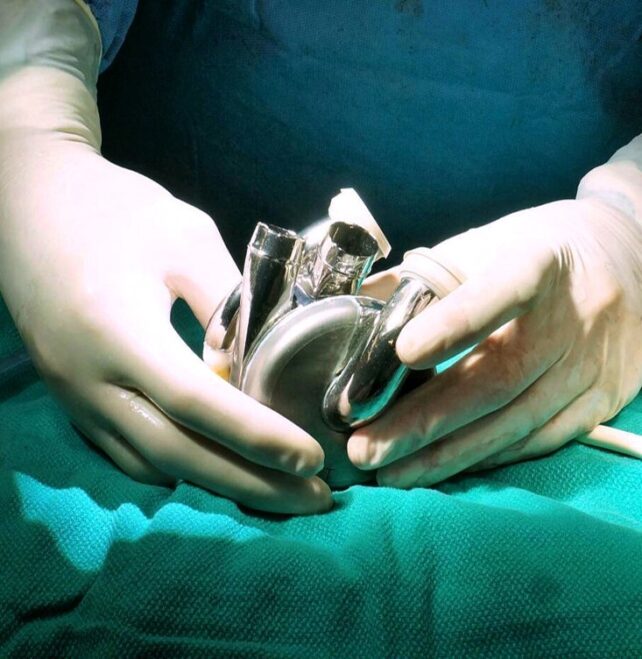
It took ten years, multiple designs, and dozens of animal studies, but the BiVACOR artificial heart finally made its way into the chest of a living patient with end-stage heart failure. The device was implanted at Baylor St. Luke's Medical Center at the Texas Heart Institute without complications.
According to the patient's doctors, the titanium heart worked very well for eight days before a real donor heart became available.
"I'm incredibly proud to witness the successful first-in-human implant of our TAH," says the founder of BiVACOR, Daniel Timms.
"This achievement would not have been possible without the courage of our first patient and their family, the dedication of our team, and our expert collaborators at The Texas Heart Institute."
Today, the best treatment option for people with severe heart failure is a real donor heart, but those aren't always available in a timely way. Each year, less than 6,000 heart transplants take place worldwide.
As such, artificial hearts are a vital way to extend and improve the quality of life for transplant-eligible patients at risk of imminent death.
In the past two decades, however, only one artificial heart has received commercial approval from the US Food & Drug Administration (FDA).
It's called the SynCardia Total Artificial Heart, and its flexing membranes and valves are quite large, and they aren't particularly durable over longer periods of time within the body.
The BiVACOR heart is celebrated by its makers as a "paradigm shift" in artificial heart design that is made to last.
No one really knows how long the device works for in humans, but on the bench top in the lab, BiVACOR's design has kept whirring away for four years and counting.
Even SynCardia's artificial heart, which is only designed for the short-term, has been known to last for years in some patients waiting to receive a transplant.

In November last year, BiVacor received FDA approval to implant its TAH in up to five patients with end-stage heart failure in 2024. Given the success of the first implant, additional transplants are expected in the near future.
"The world-wide impact of a commercially viable, long-term mechanical replacement to the failing human heart will be tremendous," reads the abstract from the ongoing clinical study.
"The wait for patients desperately requiring a heart transplant will go from years to off-the-shelf availability, thereby quickly providing a vastly improved quality of life."
